

In July 2018, China optimized the clinical trial applications (CTA) / investigational new drug (IND) applications' review and approval procedures. Instead of waiting for the formal approval notification after lengthy procedures, the applicant can start the clinical trial if there are no rejection notice or question from the Center for Drug Evaluation (CDE) within the first 60 workdays 1 from the day when the application is accepted by CDE. This is why it's called implied approval.
To help international pharma companies register drugs in China, this article introduces
Domestic applicants should be companies or drug research institutions that are registered in China and capable of taking legal responsibilities independently.
Overseas applicants should be lawful pharmaceutical companies. They should appoint corporate entities in China to act as their local agents for submitting the application.
The following drugs are permitted for clinical trial application:
Before submitting the IND application, the applicant should complete the CMC (chemistry, manufacturing, and controls) and toxicology researches to support the clinical trial. Phase I clinical trial should contain the following eight documents:
1) Table of contents for all the documents included in the application;
2) Introduction and the general plan for the research;
3) Investigator's brochure;
4) Clinical trial protocol or plan;
5) CMC research information;
6) Non-clinical research information;
7) Statement about the drug's previous clinical use;
8) Overseas research materials.
Besides, the applicant should provide the following information to CDE:
1) Situation of the pharmacovigilance system;
2) Name list of relevant parties in the clinical trial;
3) Document on the ethics committee review;
4) Document on the pre-submission communication with CDE.

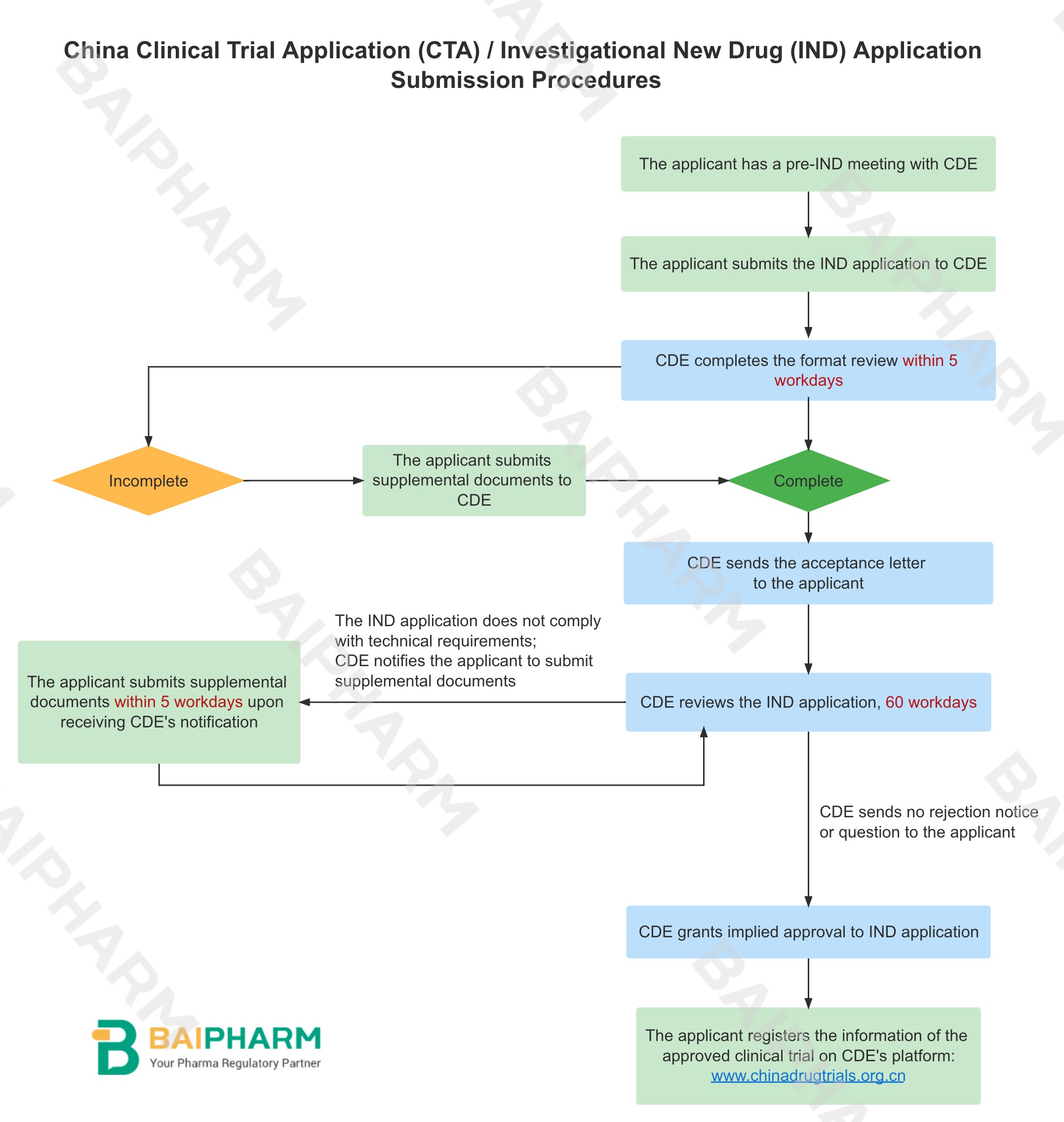
China Clinical Trial Application (CTA) / Investigational New Drug (IND) Application Procedures
As a professional regulatory compliance service provider, BaiPharm helps pharma companies submit IND applications fully compliant with CDE's regulatory requirements. Here are what we can offer:
Contact BaiPharm for pharmaceutical regulatory compliance solutions.
Related: Please refer to Part 2 of BaiPharm's article if you'd like to know on what conditions will CDE exempt clinical trials and accepts overseas clinical data.